To date there have been ten recalls of Simvastatin. They’ve all been relatively minor compared to recalls of some other popular generics, such as Lisinopril with over a million bottles recalled in one case. By contrast, the biggest Simvastatin recall so far affected just over 54,000 bottles.
The biggest recall of the popular lipid-control drug yet was the 2015 Class III recall by Micro Labs. It involved 54,096 bottles and was caused by impurities in the pills. It was actually made up of three distinct recall orders.
Finding the pertinent info on any given Simvastatin recall can be a difficult task, because of the way the FDA presents its data. Below, we’ve listed the relevant facts on all recalls of this Zocor generic, including dates, number of bottles affected, manufacturers, lot numbers, and the reasons for each recall.
Simvastatin Recalls
There have been 10 total recalls of Simvastatin through 2017. All 10 recalls were issued voluntarily by various manufacturers.
Most of the individual incidents originated from Micro Labs USA (four recalls) and Aidapak Services (three recalls). The other three came from Biocon Inc., Merck Sharp & Dohme Corp, and Attix Pharmaceuticals.
Some of the recalls were very minor. For instance, two of the Aidapak actions concerned only 90 tablets each. Half of the recalls were Class III (no immediate danger to consumers) and the other half were Class II (risk of severe injury or death, though not immediate).
The full list of all Simvastatin recalls from through 2017 is below.
Simvastatin Facts
Simvastatin is a generic form of Zocor, a statin medication first developed by Merck in the early 1990’s. The World Health Organization (WHO) considers it one of the safest, most effective, essential medications on the market. Made from the aspergillus terreus fungus, it’s used to decrease high lipid levels and manage the elevated risk of heart trouble.
Serious side effects of Simvastatin include liver problems, muscle breakdown, and high blood sugar. More common side effects are headaches, constipation, and nausea. Those with kidney trouble should take lower doses, while pregnant and nursing mothers should avoid the drug.
Major Simvastatin Recalls
The biggest two recalls of Simvastatin happened between 2014 and 2016 and involved 5,000 to 54,000 bottles each. That’s a fairly small recall count for such a popular generic. So far there have been no incidents in 2017.
The major recalls originated from Micro Labs USA and Biocon, Inc. There were a few other relatively minor recalls (less than about 1,000 bottles each), with one also from Micro Labs USA. Other companies affected by the recalls are Aidapak Services, Attix Pharmaceuticals, and Merck Sharp & Dohme Corp.
One somewhat confusing recall of the drug came from Attix Pharmaceuticals. The official FDA info on that incident states the recall quantity as “1610 grams,” but it also references all lots from 1/5/12 through 2/12/15. There’s no further info about this event on the web, but it could potentially be the biggest Simvastatin recall in existence.
Below is a bird’s eye view of all Simvastatin recalls to date. We’ve grouped distinct recalls from the same company on the same date together. For a more comprehensive view, see the sections below.
- 2016 Simvastatin recall: 5,505 bottles, Biocon Inc.
- 2015 Simvastatin recall: Single lot, Merck Sharp & Dohme Corp
- 2014 Simvastatin recall: All lots from 1/5/2012 through 2/12/2015, Attix Pharmaceuticals.
- 2014 Simvastatin recall: 1,008 bottles, Micro Labs USA.
- 2014 Simvastatin recall: 54,096 bottles, Micro Labs USA.
- 2013 Simvastatin recall: 1,180 tablets, Aidapak Services.
Simvastatin Manufacturers

Dozens of companies make Simvastatin. There’s a partial list below and a more complete list on this page.
- Lupin Pharmaceuticals
- Accord Healthcare
- Biocon
- Hetero Labs
- Dr. Reddy’s Labs
- Micro Labs USA
- Oxford Pharmaceuticals
- Mylan Pharmaceuticals
- Watson Labs
- Ivax Sub Teva Pharmaceuticals
Full List of All Simvastatin Recalls
Here’s the complete list of all 10 FDA Simvastatin recalls so far. It includes multiple recall numbers for a few of the major recalls listed above. It also gives detail on the smaller recalls, affecting less than 1,200 bottles each.
What’s the Difference Between a Class I, Class II, and Class III Recall?
Simvastatin recalls are grouped by an FDA class number. To clarify, we’ve defined the three different recall classes here.
Class I Recall. Urgent, with immediate and significant danger of death and/or serious injury. This is very rare.
Class II Recall. Serious, with non-immediate but still serious risk of death and/or injury. Half the recalls listed in this reference are Class II. This FDA recall-type is preventative in nature.
Class III Recall. This is the least serious FDA recall category. In this grouping, there’s no immediate danger from the FDA violations. Half the recalls shown below are Class III.
To date, there haven’t been any Class I recalls of Simvastatin, and there have been no recalls of the drug of any kind in 2017.
Why is “Recall Date” Different from “Issued Date” Below?
The FDA identifies recalls by three different dates: issue date, classification date, and termination date. Usually, the FDA (or a manufacturer) will order a recall on one date, then classify the severity of the recall as either Class I, II, or III at a later date. The first date is the issue date and the second is the classification date.
Below, we’ve listed all Simvastatin recalls by classification date. We’ve also listed the issue date in the first bullet point for each item.
4/8/2016 Simvastatin Recall, Class III
- Recall issued 3/24/2016.
- 5 mg Simvastatin Tablets, 90-count bottle and 1000-count bottle.
- Manufactured for Blu Pharmaceuticals.
- Reason: Subpotent Drug.
- 5505 bottles recalled.
- Lot # 14S0042F5B.
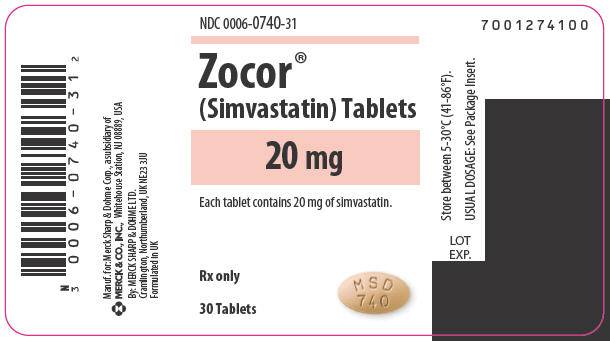
10/5/2015 Simvastatin Recall, Class II
- Recall issued 8/14/2015.
- Vytorin Tablets (each contains 20 mg Simvastatin), 1000-count bottle.
- Manufactured For Merck Sharp & Dohme Corp.
- Reason: Foreign tablets present.
- No specified number of bottles.
- Lot # L013425
4/20/2015 Simvastatin Recall, Class II
- Recall issued 11/14/2014.
- Simvastatin active pharmaceutical ingredient.
- Manufacturer: Attix Pharmaceuticals.
- Reason: Cross-contamination with penicillin.
- 1610 grams.
- All lots repackaged and distributed between 1/5/12 to 2/12/15.
11/12/2014 Simvastatin Recall, Class III
- Recall issued 8/19/2014.
- 10 mg Simvastatin Tablets, 90-count bottle.
- Manufactured for Micro Labs USA Inc.
- Reason: Failed Impurities/Degradation Specs.
- 1008 bottles.
- Lot # STBG005.
3/11/2015 Simvastatin Recall, Class III
- Recall issued 12/4/2014.
- 20 mg Simvastatin Tablets, 90-count bottle and 1000-count bottle.
- Manufactured for Micro Labs USA Inc.
- Reason: Failed Impurities/Degradation Specs.
- 240 (1000-count bottle), 26,904 (90-count bottle).
- Lot # STCG005, STCG011, STCG012.

3/11/2015 Simvastatin Recall, Class III
- Recall Issued 12/4/2014.
- 40 mg Simvastatin Tablets, 90-count bottle.
- Manufactured for Micro Labs USA Inc.
- Reason: Failed Impurities/Degradation Specs.
- 13488 bottles.
- Lot # STDG010.
3/11/2015 Simvastatin Recall, Class III
- Recall Issued 12/4/2014.
- 80 mg Simvastatin Tablets, 90-count bottle.
- Manufactured for Micro Labs USA Inc.
- Reason: Failed Impurities/Degradation Specs.
- 13464 bottles.
- Lot # STEG004, STEG006.
1/20/2014 Simvastatin Recall, Class II
- Recall Issued 7/2/2013.
- 5 mg Simvastatin Tablets.
- Manufactured for Aidapak Services, LLC.
- Reason: Label Mixup (Simvastatin Tablets possibly mislabelled as Losartan Potassium).
- 90 tablets.
- Pedigree: AD65323_13.
1/20/2014 Simvastatin Recall, Class II
- Recall Issued 7/2/2013.
- 40 mg Simvastatin Tablets.
- Manufactured for Aidapak Services, LLC.
- Reason: Label Mixup (Simvastatin Tablets possibly mislabelled as Metoprolol Tartrate).
- 90 tablets.
- Pedigree: AD22845_4.
1/16/2014 Simvastatin Recall, Class II
- Recall Issued 7/2/2013.
- 20 mg Simvastatin Tablets.
- Manufactured for Aidapak Services, LLC.
- Label Mixup (Simvastatin Tablets possibly mislabelled as Vitamin B Complex).
- 1000 tablets.
- Pedigree: W003580.
Conclusion
The list above gives the top-level data on the ten total Simvastatin recalls through 2017, including three major recalls and several minor ones. The biggest confirmed recall affected Micro Labs USA, involving 54,096 bottles in 2014. The next biggest confirmed recall affected Biocon, Inc. with 5,505 bottles. That said, there’s some confusion over the size of the Attix Pharmaceutical recall, listed by the FDA as “1610 grams” in total quantity, but affecting all lots over a period of almost three years. We’ve put a call in to the FDA for clarification as of 6/12/17.
The other recalls of this statin drug were less than 1,200 bottles each, involving Aidapak services, Micro Labs USA, and Merck Sharp & Dohme Corp.
Drug recalls can be expensive. Save money for your practice with predictable-price, predictable-service waste disposal from MedPro Waste Disposal. MedPro provides safe handling of sharps and other waste as prevention against needle sticks and other incidents. They also offer compliance training to reduce overall risk. For more about how much you can save with MedPro, see this useful savings calculator.